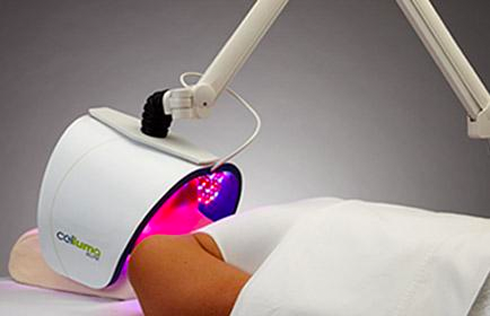
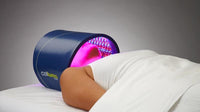
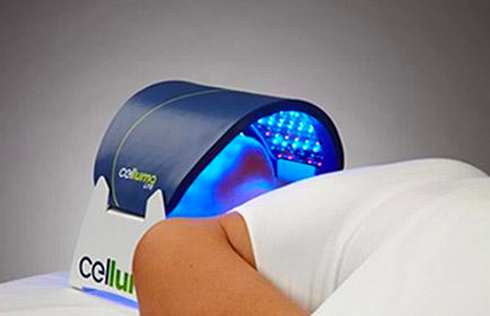
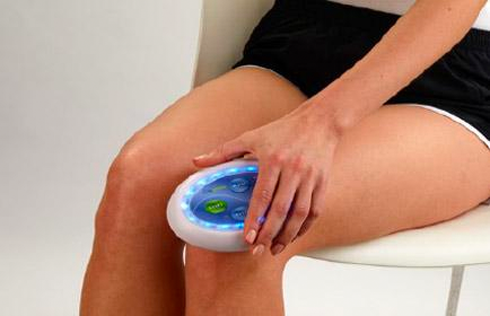
LED LIGHT THERAPY
Transform Your Cellular Health with Celluma.
What is LED Light Therapy?
So what is light emitting diode (LED) therapy? Light therapy, also known as phototherapy or low-level light therapy, is the application of specific wavelengths of light energy to tissue to obtain therapeutic benefits for a variety of conditions including skin, pain and hair restoration.
The absorbed energy is then used to improve cellular performance. Light therapy can be delivered through light emitting diode (LEDs) devices or cold LASERs, and has a variety of applications across many medical fields. This clinically validated technique is gaining greater and greater acceptance in main stream medicine by dermatologists and cosmetic surgeons as a non-invasive therapeutic modality for the treatment and healing of skin and wound conditions and by pain professionals for pain relief.
Phototherapy, or LED red light therapy, has been widely researched and is supported by thousands of peer-reviewed and published clinical research papers from prestigious institutions around the world. Other terms by which light therapy is referred to are photobiostimulation, photobiomodulation, photomedicine, LED light therapy, low-level light therapy (LLLT), red light therapy, cold and soft LASER therapy. All terms are correct, have the same meaning and are used to describe the delivery of light energy to treat a variety of medical and cosmetic conditions.
LED light therapy treatments are non-invasive, painless, require no recovery time, and can be used safely on all skin types.
The only all-in-one LED light therapy device designed to manage a variety of pain and skin conditions
In the same way that plants use chlorophyll to convert sunlight into energy, high intensity light emitting diodes (LEDs) utilizing specific, proven wavelengths of light can trigger a natural biostimulatory effect in human tissue. Research has shown that light emitting diode (LED) or phototherapy can increase circulation, accelerate tissue repair, kill acne bacteria, decrease inflammation, improve acne prone skin, skin tone, texture and clarity, decrease under eye wrinkles as well as ease muscle and joint pain, stiffness, spasm, and pain associated with arthritis.
Research indicates that cells absorb particles of light (photons) and transform their energy into adenosine triphosphate (ATP), the form of energy that cells utilize. The resulting elevation of ATP is then used to power metabolic processes; synthesize DNA, RNA, proteins, enzymes, and other products needed to repair or regenerate cell components; foster mitosis or cell proliferation; and restore homeostasis. Simply put, the LED phototherapy source provides compromised cells with added energy so the cells performance is enhanced. For example, fibroblast cells will increase collagen and elastin production in connective tissue to improve the appearance of fine lines and wrinkles in our skin and increase the rate of wound healing.
..Reported mechanisms of light-induced effects include modulation of prostaglandin levels, alteration of somatosensory evoked potential and nerve conduction velocity, and hypermia of treated tissue. 1"
Reference:
About the Celluma SERIES
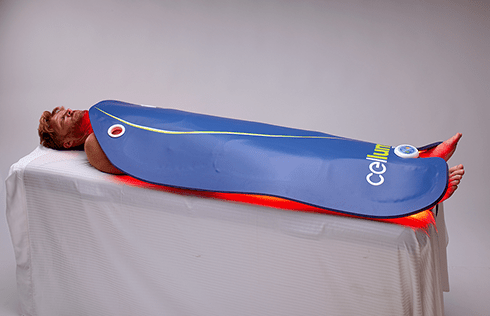
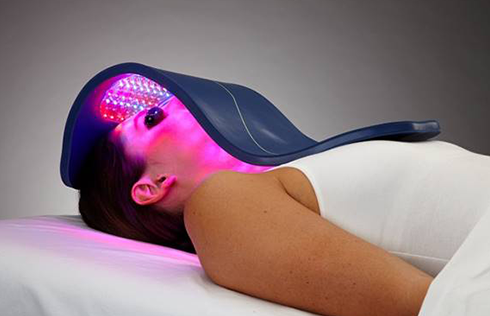
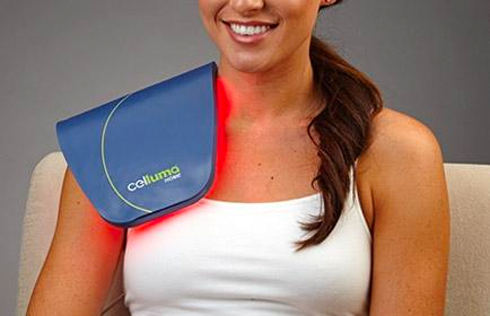
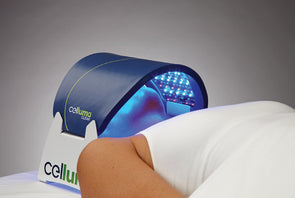
Cleared by the FDA for pain management, skin conditions and hair restoration, Celluma LED light therapy devices use specific wavelengths of light energy to improve cellular health by accelerating the repair and replenishment of compromised tissue cells for enhanced results. While similar devices on the market claim to do the same, they often require multiple machines and accessories to treat specific conditions or accommodate different parts of the body. From compact handhelds to large full-body light therapy devices, the Celluma SERIES offers convenient solutions for the treatment of skin concerns as well as muscle, joint and pain issues in a variety of versatile, affordable all-in-one systems.



Celluma Versatility
Patented, pulsed-wave technology controlled by proprietary software algorithms add to the applicability of Celluma light emitting diode therapy. With the exception of the Celluma CLEAR (emits blue and red only) for treating acne, all Celluma devices are polychromatic and deliver blue (465nm), red (640nm) and infrared (880nm) light energy simultaneously to safely treat skin, pain and hair loss easily and affordably, both in a professional setting and at home. Each wavelength is absorbed by different molecules that act as a signaling mechanism for different cellular processes. For example, some reduce inflammation and some kill acne-causing bacteria, while others enhance localized circulation. With 14 models to choose from, whether you are seeking a solution to aging skin, acneic skin, pain or hair loss, there is a Celluma model to address your concerns and budget. Most Celluma models address several conditions in a single device so be sure to choose the model that best suits your particular needs. A full description of each is available by clicking here.
Shining a Light on the Celluma Difference
When it comes to phototherapy (light emitting diode therapy), there is a broad range of optical parameters required to induce cellular responses for desired therapeutic outcomes. These specific and well-proven requirements for effective light therapy begin with choosing the correct wavelengths; blue, red and near-infrared. These are the wavelengths most widely supported by credible scientific research for the effective application of low-level light therapy. Optimal energy delivery and treatment parameters are also crucial components. The importance of device placement and proximity to tissue is one aspect of LED light therapy which is often misunderstood and rarely explained effectively. The key to efficacy is getting the light emissions as close to the skin as possible to ensure optimal energy absorption. Based on Celluma’s patented shape-taking design, no other LED device is capable of positioning better or more easily than Celluma devices. A rule of thumb is to place the device as close as possible to the skin but within your comfort level or that of the patient. Remember, the closer the device is placed to the skin the more energy is available for utilization by under-functioning cells and the faster and better the improvement to skin and pain conditions.
Based on the Inverse Square Law of optical physics, we understand that the proximity to the treatment area (skin) is key to effectiveness. This law applies to all phototherapy light devices and all brands. In order to make up for the distance factor, early LED machines were built on what is considered today to be outdated platforms, and had to resort to different strategies, including increasing the number LEDs or increasing power output. However, these tactics don’t make up for distance they are positioned from the skin, they do however add dramatically to the cost of these bulky devices. Whether treating aging skin, pain or hair conditions, the key to light therapy efficacy is not how much energy is output by a device, or how many LEDs there are, but how much energy is available for absorption by the tissue (skin). So, if using phototherapy to improve skin and pain concerns, select a shape-taking LED device such as Celluma whose patented design leverages the Inverse Square Law thereby maximizing your potential for successful treatments.

The Inverse Square Law states that certain forces on an object varies by the inverse square of the distance between the object and the source of the force. Light energy obeys this law. For example, an object placed three inches away from a light source will receive only one ninth as much energy as an object placed one inch from the light. Simply put and following the principles of the Inverse Square Law, we understand that the closer the light energy is emitted to the skin the more energy is absorbed and the faster and better you will see results. What exactly does this mean for you and how do you use this information to optimize outcomes?
Today’s advances in LED technology allows Celluma to produce devices that leverage the Inverse Square Law. Celluma devices are flexible and shape-taking in design, allowing them to be contoured closely to the treatment area whether that be facial skin, arm and leg joints or contoured over the head. In application, this means faster, better and longer lasting results for you, your clients and your patients. Essentially, placing Celluma, or any light therapy device farther away from the skin will not make it ineffective, just less effective. Therefore, it makes sense to use a shape-taking LED device which can be optimally positioned so maximum energy is absorbed in the targeted region for better and faster skin results.
Additionally, Celluma’s contouring design allows previously considered “awkward” areas such as elbows, shoulders or ankles to be treated with ease. Celluma phototherapy is hands-and stand free and does not require monitoring during the treatment session. This is a welcome advancement for Practitioners and home users alike, who can enjoy more effective skin, pain and scalp treatments with improved ease-of-use.
See how Celluma compares to other light therapy and phototherapy devices for treating skin and pain conditions. The difference is uniquely clear. Celluma is:
- Safe, clinically proven, FDA cleared and medically CE marked for skin and pain conditions
- Non-toxic, non-invasive, compared to many skin topicals
- Affordable alternative to drugs and harsh topical solutions
- Conforms closely to the treatment area for optimal effectiveness unlike other LED devices
- Patented shape-taking design. While some LED machines claim to be flexible, unlike Celluma, none can hold their shape in position without stands or cradles to hold them in place
- Treats large surface/skin areas and can be used safely anywhere on the body
- Portable and lightweight — ideal for travel and easy storage
- Takes the principles of the Inverse Square Law into account
- Embedded NASA researched technology
- Safe treatments for the entire family, including pets*
Illuminating Vitality as Nature Intended
Celluma light therapy mimics a natural photobiochemical reaction process to deliver safe, UV-free low-level light energy through FDA cleared, high-intensity LED devices. Celluma machines are FDA-cleared to treat a variety of skin and pain conditions to regain and maintain your body and skin’s natural vitality without the concern of dangerous side effects often associated with modern pharmaceuticals. Designed to address a multitude of skin and musculoskeletal conditions, Celluma is available in 1, 2 and 3-mode user-friendly devices and comes in several sizes. The Celluma DELUX permits full-body skin and pain treatments and came into being as a result of demand from customers seeking a way to treat larger areas of the body. The Celluma DELUX is medically credentialed to treat the same skin and pain conditions as other Celluma devices excluding the Celluma RESTORE which has a hair restoration program.

Depth of Light Energy Penetration

Electromagnetic Spectrum
Originally developed by NASA for astronauts who could become injured or ill on long-term space missions, low-level-light therapy is used today as a safe and natural method of treating a variety of skin and pain conditions such as acne, wrinkles, aging skin and pain.
FAQsClinical Research
See why Celluma is a preferred low-level light therapy device for wellness, pain, and skin specialists.
FDA Cleared Versus FDA Approved?
Have you ever wondered about the difference between FDA Cleared versus FDA Approved? Read on for a full explanation. The FDA regulates all medical claims made by device manufacturers regarding their products. The Agency uses two different processes depending on comparative risk for reviewing medical claims before manufacturers are permitted to make those claims commercially.
The 510(k) Process
The 510 (k) process is intended to review devices that are better understood by the Agency and/or potentially less hazardous to patients, like the Celluma. This process is most often used to review Class II devices. This is a less burdensome review process and if the Agency concludes that the manufacturer has demonstrated that the device is substantially equivalent to a legally marketed device, the device is “cleared” for commercialization. A device being “cleared” is not reflection on the efficacy of the device, only an indication of the comparative risk in use.
The Pre-Market Approval (PMA) process
The Pre-Market Approval (PMA) process is intended for devices not as well known to the Agency and that pose a greater risk to the patient, like implantable devices. An example would be a heart stint. These types of products are considered Class III devices. A PMA is a more burdensome process and if the Agency concludes that the manufacturer’s claims are supported using this process, the device is “approved” for commercialization. As a particular Class III device becomes better known to the FDA, it may be re-classified as a Class II device, meaning any subsequent review of such devices by the Agency will be done using the 510(k) process. Again, a PMA “approval” does not denote the relative efficacy of a device, only the comparative risk of the device in use.
That said, a device “clearance” is not somehow a subordinated “rating” by the Agency in comparison to an “approval”. It is just the favorable conclusion of a different review processes used by the Agency, depending on how common and how risky the device is getting reviewed.
Competitor Analysis
| Device Feature | CELLUMA | Dermalux Flex MD |
|---|---|---|
| Wavelengths | Blue, Red, Near Infrared* | Blue, Red, Near Infrared* |
| Treatment Time | Up to 30 minutes | Up to 30 minutes |
| Application | Face & Body | Face & Body |
| Number of LEDs | 356 | 360 |
| Dose (fluence) J/cm² (in typical facial position) | 0.449-0.877 J/cm² | 0.178-0.313 J/cm² |
| Treatment Area | 718.5 cm² | 730 cm² |
| Hygiene Barriers + Face Shields | ||
| In-Person and Online Certification (Up to Level 4 and 60 CPD points) | ||
| Marketing Support | ||
| Manufacture | USA | UK |
| Medical CE | ||
| Globally patented shape-taking design | ||
| Product Innovation of the Year | WINNER | |
| FDA-cleared on OTC basis |